38 what term is used to label the energy levels of electrons
How Many Electrons Can Each Energy Level Hold? - Reference.com The maximum number of electrons that an energy level can hold is determined from the formula 2n^2 equals the total number, where n is the energy level. Thus, the first energy level holds 2 * 1^2 = 2 electrons, while the second holds 2 * 2^2 = 8 electrons. Following the formula, the third energy level can contain 18 electrons, the fourth energy ... PDF Electrons and The Structure of Atoms - Jefferson Academy Chemistry Circle the letter of the term that is used to label the energy levels of electrons. a. atomic orbitals c. quantum b. quantum mechanical numbers d. principal quantum numbers (n) 10. The letter is used to denote a spherical orbital. 11. Label each diagram below p x, p y, or p z. 12. Use the diagram above. Describe how the p x, p y, and p z ...
What is the term used to label the energy levels of electrons? - Answers What term is used to label energy levels of electrons? Principal quantum numbers (n). What is the term valance band? It refers to the energy levels in an atom where the electrons that participate...
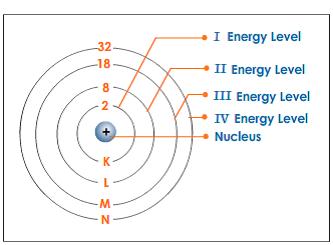
What term is used to label the energy levels of electrons
How to Represent Electrons in an Energy Level Diagram You look on the periodic table and find that oxygen is atomic number 8. This number means that oxygen has 8 protons in its nucleus and 8 electrons. So you put 8 electrons into your energy level diagram. You can represent electrons as arrows. If two electrons end up in the same orbital, one arrow faces up and the other faces down. Atomic Structure: The Bohr Model - dummies Bohr used the term energy levels (or shells) to describe these orbits of differing energy. He said that the energy of an electron is quantized, meaning electrons can have one energy level or another but nothing in between. The energy level an electron normally occupies is called its ground state. But it can move to a higher-energy, less-stable ... Energy levels | Article about Energy levels by The Free Dictionary In X-ray spectroscopy the rydberg (Ry) is used as the unit of energy; 1 Ry = 13.606 eV. In optical spectroscopy we often speak of the spectral term, which means the value of T = -ℰ/ hc. For atoms, T is reckoned from the ionization limit and is given in cm -1. M. A. E L'IASHEVICH
What term is used to label the energy levels of electrons. Definition and Classification of Energy Bands | Band Theory - BYJUS The electrons in the same orbit exhibit different energy levels. The grouping of this different energy levels is known as energy band. However, the energy of the inner orbit electrons are not much affected by the presence of neighbouring atoms. Classification of Energy Bands Valence Band Solved Molecule of study: K3[Co(C2O4)3] a) Write down the - Chegg c) Use orbital and symmetry symbols to label energy levels and assign electrons to the energy levels in your diagram, considering that this compound is paramagnetic. [5 marks] d) Clearly identify the Δo energy splitting transition on your MO diagram and explain your choice. Calculate the splitting energy in cm-1 knowing that λmax = 600 nm. [5 ... Solved Atomic Physics 1. Draw and label an energy level | Chegg.com Science. Advanced Physics. Advanced Physics questions and answers. Atomic Physics 1. Draw and label an energy level diagram for hydrogen using the Bohr Model. On it, show all the transitions by which an electron in the n 4 state could emit a photon. Question: Atomic Physics 1. atom - Orbits and energy levels | Britannica The outermost shell of electrons—called the valence shell—determines the chemical behaviour of an atom, and the number of electrons in this shell depends on how many are left over after all the interior shells are filled. periodic table showing the valence shells The periodic table of the elements showing the valence shells.
What term is used to label the energy levels of electrons? - Answers What term is used to label energy levels of electrons? Principal quantum numbers (n). What is the term valance band? It refers to the energy levels in an atom where the electrons that participate... Energy Level of an Atom - VEDANTU The first energy level is also called level 'K'. The second level is called level L, third energy level as M, and so on. The electrons from energy level K contains the least energy whereas the levels that are far from the nucleus contains more energy Electrons in the outermost energy level are also called Valence electrons. Chapter 5 test Section 5.1 Flashcards | Quizlet The term that is used to label the energy levels of electrons is? S What letter is used to denote a spherical orbital? Dumbell All "p" orbital's are _____ shaped? 2n^2 What is the formula for the maximum number of electrons that can occupy a principal energy level? (Use "n" for the principal quantum number) Recommended textbook explanations s,p,d,f Orbitals - Chemistry | Socratic An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. The order of size is 1s < 2s < 3s < …, as shown below.
Chapter 5 Chem Flashcards | Quizlet Quantum of energy is the amount of energy required to Farther the higher the electron the blank it is from the nucleus Atomic Orbital often thought of as a region of space in which there is a high probability of finding an electron Principle quantum numbers the term used to label the energy levels of electrons S used to denotate a spherical orbital Protons, Neutrons, and Electrons - Middle School Chemistry It shows the electron in the space surrounding the nucleus that is called an electron cloud or energy level. It is not possible to know the location of an electron but only the region where it is most likely to be. The electron cloud or energy level shows the region surrounding the nucleus where the electron is most likely to be. Energy level diagram for Molecular orbitals - Class Notes 2) Stability of molecules in terms of bond order. Bond order is defined as half of the difference between the number of electrons present in the bonding and antibonding orbitals.. Bond Order = ½ ( N b - Na). The molecule is stable if N b > Na ie. bond order is positive. The molecule is unstable if N b < Na i.e. the bond order is negative or zero. 3) Relative stability of molecule in terms ... What term is used to label the energy levels of electrons? The term that is used to label the energy levels of electrons are principle quantum numbers and a valance band refers to the "energy levels in an atom where the electrons that participate in bonding occupy. These energy levels correspond to those of the s and p orbitals of the outermost shell of the atom being considered." Hope this helps! :)
6.4 Electronic Structure of Atoms (Electron Configurations) The n = 1 shell is filled with two electrons and three electrons will occupy the n = 2 shell. Because any s subshell can contain only two electrons, the fifth electron must occupy the next energy level, which will be a 2 p orbital. There are three degenerate 2 p orbitals ( ml = −1, 0, +1) and the electron can occupy any one of these p orbitals.
What Is the Maximum Number of Electrons in Each Energy Level? The maximum number of electrons found on energy levels one through six are two, eight, 18, 32, 50 and 72. The formula for determining the number of electrons is two multiplied by n squared, or 2n^2. The energy levels are typically referred to by their shell number rather than their energy level. In ascending order, the shell letters are K, L, M ...
Atomic Energy Levels (video) - Khan Academy All that matters is what energy level or rung on the ladder the electron is at. Note that the electron for our hypothetical atom here can only exist with zero eV, four, six, or seven eV. The electron just cannot exist between energy levels. It's always got to be right on one of the energy levels.
Energy Level Diagram - Different Energy Shells Around the Nucleus What is an energy level diagram? In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. Bohr developed this model of the atom which says the electrons revolve around the nucleus in a circular path called an orbit.
Energy Level and Transition of Electrons - Brilliant In chemistry, energy is a measure of how stable a substance is. The lower the energy level of an electron, the more stable the electron is. Thus an electron would be in its most stable state when it is in the K shell (n=1). (n = 1). For this reason, we refer to n=1 n = 1 as the ground state of the electron.
electrons and energy. - Brooklyn College Energy and Electrons: ... Levels of Energy: All the research on atomic structure and the hideously difficult-to-understand properties of electrons come together in the topic of "electron energy". An atom such as lithium has three electrons in various orbitals surrounding the atomic center. These electrons can be bombarded with energy and if ...
PDF Electron Energy and Light Key energy level 5 to energy level 2. Refer to Models I and 2 for the following questions. a. Label the picture with "n=5 to n=2" and list the corresponding color of light emitted. b. This electron transition (absorbs e eases) ergy. c. This electron moves from a (lower/ gher) ery state to (lower igher) energy state.
Energy level - Wikipedia The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be quantized .
The periodic table, electron shells, and orbitals - Khan Academy By convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. In order to move between shells, an electron must absorb or release an amount of energy corresponding exactly to the difference in energy between the shells.
Electron arrangement - What does the periodic table tell ... - BBC Bitesize and so 11 electrons. 2 electrons occupy the first shell; 8 electrons occupy the second shell; 1 electron occupies the third shell; This electron arrangement can be written as 2.8.1 (each dot ...
Energy levels | Article about Energy levels by The Free Dictionary In X-ray spectroscopy the rydberg (Ry) is used as the unit of energy; 1 Ry = 13.606 eV. In optical spectroscopy we often speak of the spectral term, which means the value of T = -ℰ/ hc. For atoms, T is reckoned from the ionization limit and is given in cm -1. M. A. E L'IASHEVICH
Atomic Structure: The Bohr Model - dummies Bohr used the term energy levels (or shells) to describe these orbits of differing energy. He said that the energy of an electron is quantized, meaning electrons can have one energy level or another but nothing in between. The energy level an electron normally occupies is called its ground state. But it can move to a higher-energy, less-stable ...
How to Represent Electrons in an Energy Level Diagram You look on the periodic table and find that oxygen is atomic number 8. This number means that oxygen has 8 protons in its nucleus and 8 electrons. So you put 8 electrons into your energy level diagram. You can represent electrons as arrows. If two electrons end up in the same orbital, one arrow faces up and the other faces down.





























:max_bytes(150000):strip_icc()/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)

Post a Comment for "38 what term is used to label the energy levels of electrons"